|
|||||||||
Gold
Gold is a metal with the symbol Au (from Latin: aurum). It has been a highly sought-after precious metal for coinage, jewelry, and other arts since the beginning of recorded history. The metal occurs as nuggets or grains in rocks, in veins and in alluvial deposits. Gold is dense, soft, shiny and the most malleable and ductile pure metal known. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Gold is one of the coinage metals and has served as a symbol of wealth and a store of value throughout history. Although primarily used as a store of value, gold has traditionally found use because of its good resistance to oxidative corrosion and excellent quality as a conductor of electricity. Gold is prized for its beauty. Jewelers and metalsmiths value it as a metal that can be embossed, hammered, cast, stretched or twisted. Compared with other metals, pure gold is chemically least reactive. Gold dissolves in mercury, forming amalgam alloys, but does not react with it. Gold is insoluble in nitric acid, which dissolves silver and base metals. This property is exploited in the gold refining technique known as "inquartation and parting". Nitric acid has long been used to confirm the presence of gold in items, and this is the origin of the colloquial term "acid test", referring to a gold standard test for genuine value. Jacques Blanc “Gold on Skin”Jacques Blanc “Gold on skin” is a new concept of a luxury brand. Ephemeral and precious. “Gold on Skin” was launched in Paris, at Colette’s in 2010. Mainly pure 24K gold is used. We offer also a range of colored golds -over 90% of pure gold and non-toxic, non-allergenic metals. Laid on skin by artists, the patterns are original and unique. Jacques Blanc specialises in pure gold. Other services : gold on cars and bespoke interiors. Regarding bespoke Interiors, we do not use standard gold leaves, but special small 24K loose gold leaves we let move. It’s different.. Characteristics of goldGold is the most malleable and ductile of all metals; a single gram can be beaten into a thin sheet. Gold leaf can be beaten thin enough to become translucent. The transmitted light appears greenish blue, because gold strongly reflects yellow and red. Gold readily creates alloys with many other metals. These alloys can be produced to modify the hardness and other metallurgical properties, to control melting point or to create exotic colors. Gold is a good conductor of heat and electricity and reflects infrared radiation strongly. Chemically, it is unaffected by air, moisture and most corrosive reagents, and is therefore well suited for use in coins and jewelry as well as skin jewels and as a protective coating on other. High quality pure metallic gold is tasteless and scentless; in keeping with its resistance to corrosion. In addition, gold is very dense, a cubic meter weighing 19,300 kg. By comparison, the density of lead is 11,340 kg/m3, and that of the densest element, osmium, is 22,610 kg/m3. A pack of cigarettes full of gold would weight more than 2kg. Colored goldWhereas most other pure metals are gray or silvery white, gold is yellow . As while pure gold -designated as 24k.- is yellow in color, colored gold can be developed into various colors. These colors are generally obtained by alloying gold with other elements in various proportions. The gold content of alloys is measured in carats (K). Pure gold is designated as 24K. For example, alloys which are mixed 14 parts gold to 10 parts alloy create 14-karat gold, 18 parts gold to 6 parts alloy creates 18 karat, and so on. This is often expressed as the result of the ratio, i.e.: 14/24 equals 0.585 and 18/24 is 0.750. There are hundreds of possible alloys and mixtures, but in general the addition of silver will color gold white, and the addition ofcopper will color it red. A mix of around 50/50 copper and silver gives the range of yellow gold alloys the public is accustomed to seeing in the marketplace. A small amount (0.2%) of zinc can be added to harden the alloy. The most common grades of gold, in addition to pure 24K, are 22K (92%), 18K (75%), 14K (58%) and 9K (38%). Jacques Blanc’s Pure Gold on Skin jewels use pure 24K gold.
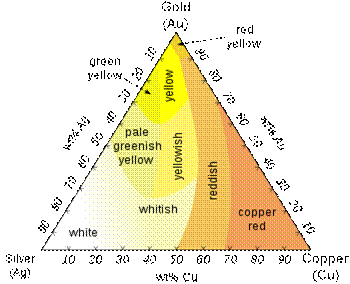
Ternary plot of different colors of Ag-Au-Cu alloys White goldWhite gold is an alloy of gold and at least one white metal, usually nickel, manganese or palladium. Like yellow gold, the purity of white gold is given in carats. White gold's properties vary depending on the metals and proportions used. As a result, white gold alloys can be used for different purposes; while a nickel alloy is hard and strong and therefore good for rings and pins, gold-palladium alloys are soft, pliable and good for white gold gemstone settings. Jacques Blanc’s godl on skin jewels can also be available in Lunar White Gold. The term white gold is used very loosely in the industry to describe karat gold alloys with a whitish hue. Many believe that the color of the rhodium plating, which is seen on many commercial pieces, is actually the color of white gold. The term "white" covers a large spectrum of colors that borders or overlaps pale yellow, tinted brown, and even very pale rose. The jewelry industry often hides these off-white colors by rhodium plating. A common white gold formulation consists of 90 wt.% gold and 10 wt.% nickel. Copper can be added to increase malleability. The alloys used in jewelry industry are gold-palladium-silver and gold-nickel-copper-zinc. Palladium and nickel act as primary bleaching agents for gold; zinc acts as a secondary bleaching agent to attenuate the color of copper. Jacques Blanc’s Lunar White Gold contains 95 wt.% gold and 5 wt.% palladium. Contact allergyAbout one person in eight has a mild allergic reaction to the nickel in some white gold alloys when worn over long periods. A typical reaction is a minor skin rash. White gold alloys made without nickel are less likely to be allergenic. Rose, red, and pink goldRose gold is a gold and copper alloy widely used for specialized jewelry. It is also known as pink gold and red gold. As it was popular in Russia at the beginning of the nineteenth century, it is also known as Russian gold, although this term has become somewhat rare. Although the names are often used interchangeably, the difference between red, rose, and pink gold is the copper content – the higher the copper content, the stronger the red coloration. A common alloy for rose gold is 75% gold and 25% copper by mass (18 karat). Since rose gold is an alloy, there is no such thing as "pure rose gold". A common formulation for red gold is 50% gold and 50% copper. Up to 15% zinc can be added to copper-rich alloys to change their color to reddish yellow or dark yellow. Rose gold alloys : The highest karat version of rose gold is also known as crown gold, which is 22 karat. Eighteen karat red gold may be made of 25% copper and 75% gold. For 18 karat rose gold, typically about 4% silver is added to the 75% gold and 21% copper to give a rose color. 14 karat red gold is often found in the Middle East and contains 41.67% copper.Green goldGreen gold alloys are made by leaving the copper out of the alloy mixture and just using gold and silver. It actually appears as a greenish yellow rather than green. Eighteen karat green gold would therefore contain a mix of gold 75% and silver 25% (or 73% gold and 27% silver).Fired enamels adhere better to these alloys. Jacques Blanc’s Absolute Green Gold contains a mix of gold 90% and silver 10%. Green gold was known to Lydians as long ago as 860 BC under the name electrum. Electrum is a naturally occurring alloy of silver and gold. Cadmium can be added to gold alloys in amount of up to 4% to achieve green color. The alloy of 75% gold, 23% copper, and 2% cadmium yields light green 18ct gold. The alloy of 75% gold, 15% silver, 6% copper, and 4% cadmium yields a dark green alloy. Cadmium is however toxic. Grey goldGrey gold alloys are made by adding silver, manganese and copper in specific ratios to the gold. Black goldBlack gold is a type of gold used in jewelry. Black colored gold can be produced by various methods:
A range of colors from brown to black can be achieved on copper-rich alloys by treatment with potassium sulfide.[2] More recently a laser technique has been developed that renders the surface of metals deep black. A femtosecond laser pulse deforms the surface of the metal forming nanostructures. The immensely increased surface area can absorb virtually all the light that falls on it thus rendering it deep black.[8] Purple and blue goldsPurple gold (also called amethyst gold and violet gold) is an alloy of gold and aluminum rich in gold-aluminium intermetallic (AuAl2). Gold content in AuAl2 is around 79% and can therefore be referred to as 18 karat gold. Purple gold is more brittle than other gold alloys, as it is an intermetallic compound instead of a malleable alloy, and a sharp blow may cause it to shatte. It is therefore usually machined and faceted to be used as a "gem" in conventional jewelry rather than by itself. At lower content of gold, the material is composed of the intermetallic and an aluminium-rich solid solution phase. At higher content of gold, the gold-richer intermetallic AuAl forms; the purple color is preserved to about 15% of aluminium. At 88% of gold the material is composed of AuAl and changes color. Blue gold is an alloy of gold and indium. It contains 46% gold (about 11 ct) and 54% indium, forming an intermetallic compound, with a clear blue color. With gallium, gold forms an intermetallic (58.5% Au, 14ct) which has slight bluish hue. All these intermetallics are brittle. A small amount of palladium, copper or silver can be added to achieve a less brittle microstructure. The intermetallic compounds tend to have poor corrosion resistance. The less noble elements are leached to the environment, and a gold-rich surface layer is formed. Direct contact of blue and purple gold elements with skin should be avoided as exposition to sweat may result in metal leaching and discoloration of the metal surface. A surface plating of blue gold on karat gold or sterling silver can be achieved by a gold plating of the surface, followed by indium plating, with layer thickness matching the 1:2 atomic ratio. A heat treatment then causes interdiffusion of the metals and formation of the required intermetallic compound. History
Funerary mask of Tutankhamun Gold has been known and used by artisans since the Chalcolithic. Gold artifacts in the Balkans appear from the 4th millennium BC, such as that found in the Varna Necropolis. Gold artifacts such as thegolden hats and the Nebra disk appeared in Central Europe from the 2nd millennium BC Bronze Age. Egyptian hieroglyphs from as early as 2600 BC describe gold, which king Tushratta of the Mitanni claimed was "more plentiful than dirt" in Egypt. Egypt and especially Nubia had the resources to make them major gold-producing areas for much of history. The earliest known map is known as the Turin Papyrus Map and shows the plan of a gold mine in Nubia together with indications of the local geology. The primitive working methods are described by both Strabo and Diodorus Siculus, and included fire-setting. Large mines were also present across the Red Sea in what is now Saudi Arabia. The legend of the golden fleece may refer to the use of fleeces to trap gold dust from placer deposits in the ancient world. Gold is mentioned frequently in the Old Testament, starting with Genesis 2:11 (at Havilah) and is included with the gifts of the magi in the first chapters of Matthew New Testament. The Book of Revelation 21:21 describes the city of New Jerusalem as having streets "made of pure gold, clear as crystal". The south-east corner of the Black Sea was famed for its gold. Exploitation is said to date from the time of Midas, and this gold was important in the establishment of what is probably the world's earliest coinage in Lydia around 610 BC. From the 6th or 5th century BC, the Chu (state) circulated the Ying Yuan, one kind of square gold coin. In Roman metallurgy, new methods for extracting gold on a large scale were developed by introducing hydraulic mining methods, especially in Hispania from 25 BC onwards and in Dacia from 106 AD onwards. One of their largest mines was at Las Medulas in León (Spain), where seven long aqueducts enabled them to sluice most of a large alluvial deposit. The mines at Roşia Montană in Transylvania were also very large, and until very recently, still mined by opencast methods. They also exploited smaller deposits in Britain, such as placer and hard-rock deposits at Dolaucothi. The various methods they used are well described by Pliny the Elder in his encyclopedia Naturalis Historia written towards the end of the first century AD. The Mali Empire in Africa was famed throughout the old world for its large amounts of gold. Mansa Musa, ruler of the empire (1312–1337) became famous throughout the old world for his great hajj to Mecca in 1324. When he passed through Cairo in July 1324, he was reportedly accompanied by a camel train that included thousands of people and nearly a hundred camels. He gave away so much gold that it depressed the price in Egypt for over a decade. The European exploration of the Americas was fueled in no small part by reports of the gold ornaments displayed in great profusion by Native American peoples, especially in Central America, Peru, Ecuador and Colombia. The Aztecs regarded gold as literally the product of the gods, calling it "god excrement" (teocuitlatl in Nahuatl). Some of Jacques Banc’s patterns are inspired of Inca’s jewels patterns. Although the price of some platinum group metals can be higher, gold has long been considered the most desirable of precious metals, and its value has been used as the standard for many currencies (known as the gold standard) in history. Gold has been used as a symbol for purity, value, royalty, and particularly roles that combine these properties. Gold as a sign of wealth and prestige was ridiculed by Thomas More in his treatise Utopia. On that imaginary island, gold is so abundant that it is used to make chains for slaves, tableware and lavatory-seats. When ambassadors from other countries arrive, dressed in ostentatious gold jewels and badges, the Utopians mistake them for menial servants, paying homage instead to the most modestly dressed of their party. There is an age-old tradition of biting gold to test its authenticity. Although this is certainly not a professional way of examining gold, the bite test should score the gold because gold is a soft metal, as indicated by its score on the Mohs' scale of mineral hardness. The purer the gold the easier it should be to mark it. Painted lead can cheat this test because lead is softer than gold (and may invite a small risk of lead poisoning if sufficient lead is absorbed by the biting). Gold in antiquity was relatively easy to obtain geologically; however, 75% of all gold ever produced has been extracted since 1910. One main goal of the alchemists was to produce gold from other substances, such as lead — presumably by the interaction with a mythical substance called the philosopher's stone. Although they never succeeded in this attempt, the alchemists promoted an interest in what can be done with substances, and this laid a foundation for today's chemistry. Their symbol for gold was the circle with a point at its center (☉), which was also the astrological symbol and the ancient Chinese character for the Sun. For modern creation of artificial gold by neutron capture, see gold synthesis. During the 19th century, gold rushes occurred whenever large gold deposits were discovered. The first documented discovery of gold in the United States was at the Reed Gold Mine near Georgeville, North Carolina in 1803. The first major gold strike in the United States occurred in a small north Georgia town called Dahlonega. Further gold rushes occurred in California, Colorado, Otago in New Zealand, Australia, Witwatersrand in South Africa, and the Klondike in Canada. Because of its historically high value, much of the gold mined throughout history is still in circulation in one form or another. SymbolismGold has been highly valued in many societies throughout the ages. In keeping with this it has often had a strongly positive symbolic meaning closely connected to the values held in the highest esteem in the society in question. Gold may symbolize power, strength, wealth, warmth... Great human achievements are frequently rewarded with gold, in the form of gold medals, goldentrophies and other decorations. Winners of athletic events and other graded competitions are usually awarded a gold medal. Many awards such as the Nobel Prize are made from gold as well. Other award statues and prizes are depicted in gold or are gold plated (such as the Academy Awards, the Golden Globe Awards, the Emmy Awards, the Palme d'Or, and the British Academy Film Awards). Aristotle in his ethics used gold symbolism when referring to what is now commonly known as the "golden mean". Similarly, gold is associated with perfect or divine principles, such as in the case of Phi, which is sometimes called the "golden ratio". Gold represents great value. Respected people are treated with the most valued rule, the "golden rule". A company may give its most valued customers "gold cards" or make them "gold members". We value moments of peace and therefore we say: "silence is golden". In Greek mythology there was the "golden fleece". Gold is further associated with the wisdom of aging and fruition. The fiftieth wedding anniversary is golden. Our precious latter years are sometimes considered "golden years". The height of a civilization is referred to as a "golden age". In Christianity gold has sometimes been associated with the extremities of utmost evil and the greatest sanctity. In the Book of Exodus, the Golden Calf is a symbol of idolatry. In the Book of Genesis, Abraham was said to be rich in gold and silver, and Moses was instructed to cover the Mercy Seat of the Ark of the Covenant with pure gold. In Christian art the halos of Christ, Mary and the Christian saints are golden. Medieval kings were inaugurated under the signs of sacred oil and a golden crown, the latter symbolizing the eternal shining light of heaven and thus a Christian king's divinely inspired authority. Wedding rings have long been made of gold. It is long lasting and unaffected by the passage of time and may aid in the ring symbolism of eternal vows before God and/or the sun and moon and the perfection the marriage signifies. In Orthodox Christianity, the wedded couple is adorned with a golden crown during the ceremony, an amalgamation of symbolic rites. In popular culture gold holds many connotations but is most generally connected to terms such as good or great, such as in the phrases: "has a heart of gold", "that's golden!", "golden moment", "then you're golden!" and "golden boy". Gold also still holds its place as a symbol of wealth and through that, in many societies, success. OccurrenceGold's atomic number of 79 makes it one of the higher atomic number elements which occur naturally. Like all elements with atomic numbers larger than iron, gold is thought to have been formed from a supernova nucleosynthesis process. Their explosions scattered metal-containing dusts (including heavy elements like gold) into the region of space in which they later condensed into our solar system and the Earth. On Earth, whenever elemental gold occurs, it appears most often as a metal solid solution of gold with silver, i.e. a gold silver alloy. Such alloys usually have a silver content of 8–10%. Electrum is elemental gold with more than 20% silver. Electrum's color runs from golden-silvery to silvery, dependent upon the silver content. The more silver, the lower the specific gravity. Gold is found in ores made up of rock with very small or microscopic particles of gold. Native gold is also found in the form of free flakes, grains or larger nuggets that have been eroded from rocks and end up in alluvial deposits (calledplacer deposits). Such free gold is always richer at the surface of gold-bearing veins owing to theoxidation of accompanying minerals followed by weathering, and washing of the dust into streams and rivers, where it collects and can be welded by water action to form nuggets. The world's oceans contain gold. A number of people have claimed to be able to economically recover gold from sea water, but so far they have all been either mistaken or crooks. A so-called reverend, Prescott Jernegan ran a gold-from-seawater swindle in the United States in the 1890s. A British fraudster ran the same scam in England in the early 1900s. Fritz Haber (the German inventor of the Haber process) did research on the extraction of gold from sea water in an effort to help pay Germany's reparations following World War I. Based on the published values of 2 to 64 ppb of gold in seawater a commercially successful extraction seemed possible. After analysis of 4000 water samples yielding an average of 0.004 ppb it became clear that the extraction would not be possible and he stopped the project. No commercially viable mechanism for performing gold extraction from sea water has yet been identified. Gold synthesis is not economically viable and is unlikely to become so in the foreseeable future ProductionGold extraction is most economical in large, easily mined deposits. Since the 1880s, South Africa has been the source for a large proportion of the world's gold supply, with about 50% of all gold ever produced having come from South Africa. Production in 1970 accounted for 79% of the world supply, producing about 1,480 tonnes. 2008 production was 2,260 tonnes. In 2007 China (with 276 tonnes) overtook South Africa as the world's largest gold producer, the first time since 1905 that South Africa has not been the largest. The city of Johannesburg located in South Africa was founded as a result of the Witwatersrand Gold Rush which resulted in the discovery of some of the largest gold deposits the world has ever seen. Gold fields located within the basin in the Free State and Gauteng provinces are extensive in strike and dip requiring some of the world's deepest mines, with the Savuka and TauTona mines being currently the world's deepest gold mine at 3,777 m. The Second Boer War of 1899–1901 between the British Empire and the AfrikanerBoers was at least partly over the rights of miners and possession of the gold wealth in South Africa. Other major producers are the United States, Australia, Russia and Peru. Mines in South Dakota and Nevada supply two-thirds of gold used in the United States. In South America, the controversial project Pascua Lama aims at exploitation of rich fields in the high mountains of Atacama Desert, at the border between Chile and Argentina. Today about one-quarter of the world gold output is estimated to originate from artisanal or small scale mining. After initial production, gold is often subsequently refined industrially by the Wohlwill processwhich is based on electrolysis or by the Miller process, that is chlorination in the melt. The Wohlwill process results in higher purity, but is more complex and is only applied in small-scale installations. Other methods of assaying and purifying smaller amounts of gold include parting and inquartation as well as cupellation, or refining methods based on the dissolution of gold in aqua regia. At the end of 2009, it was estimated that all the gold ever mined totaled 165,000 tonnes. This can be represented by a cube with an edge length of about 20.28 meters. The average gold mining and extraction costs were about US$ 317/oz in 2007, but these can vary widely depending on mining type and ore quality; global mine production amounted to 2,471 tonnes. Gold is so stable and so valuable that almost all gold used in manufactured goods, jewelry, and works of art is eventually recovered and recycled. Some gold used in spacecraft and electronic equipment cannot be profitably recovered, but it is generally used in these applications in the form of extremely thin layers or extremely fine wires so that the total quantity used (and lost) is small compared to the total amount of gold produced and stockpiled. Thus there is no true consumption of gold in the economic sense; the stock of gold remains essentially constant while ownership shifts from one party to another. ConsumptionIndia is the world's largest consumer of gold, as Indians buy about 25% of the world's gold, purchasing approximately 800 tonnes of gold every year. India is also the largest importer of the yellow metal; in 2008, India imported around 400 tonnes of gold. Price
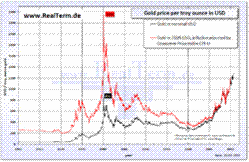
Gold price per troy ounce in USD since 1960, in nominal US$ (black) and in 2009 US$ (red) after inflation adjustment using the CPI-U price index. Like other precious metals, gold is measured by troy weight and by grams. When it is alloyed with other metals the term carat or karat is used to indicate the amount of gold present, with 24 carats being pure gold and lower ratings proportionally less. The purity of a gold bar or coin can also be expressed as a decimal figure ranging from 0 to 1, known as the millesimal fineness, such as 0.995 being very pure. The price of gold is determined through trading in the gold and derivatives markets, but a procedure known as the Gold Fixing in London, originating in September 1919, provides a daily benchmark price to the industry. The afternoon fixing was introduced in 1968 to provide a price when US markets are open. Historically gold coinage was widely used as currency; when paper money was introduced, it typically was a receipt redeemable for gold coin or bullion. In an economic system known as the gold standard, a certain weight of gold was given the name of a unit of currency. For a long period, the United States government set the value of the US dollar so that one troy ounce was equal to $20.67 ($664.56/kg), but in 1934 the dollar was devalued to $35.00 per troy ounce ($1125.27/kg). By 1961, it was becoming hard to maintain this price, and a pool of US and European banks agreed to manipulate the market to prevent further currency devaluation against increased gold demand. Central banks still hold historical gold reserves as a store of value although the level has generally been declining. The largest gold depository in the world is that of the U.S. Federal Reserve Bank in New York, which holds about 3% of the gold ever mined, as does the similarly laden U.S. Bullion Depository at Fort Knox. Since 1968 the price of gold has ranged widely, from a high of $850/oz on January 21, 1980, to a low of $252.90/oz on June 21, 1999 (London Gold Fixing). The period from 1999 to 2001 marked the "Brown Bottom" after a 20-year bear market. Prices increased rapidly from 1991, but the 1980 high was not exceeded until January 3, 2008 when a new maximum of $865.35 per troy ounce was set. Another record price was set on March 17, 2008 at $1023.50/oz. In the fall of 2009, gold markets experienced renewed momentum upwards due to increased demand and a weakening US dollar. On December 2, 2009, Gold passed the important barrier of US$1200 per ounce to close at $1215. Gold further rallied hitting new highs. Since April 2001 the gold price has more than tripled in value against the US dollar, prompting speculation that this long secular bear market has ended and a bull market has returned. Use and applicationsJewelryBecause of the softness of pure 24k gold, it is usually alloyed with base metals for use in jewelry, altering its hardness and ductility, melting point, color and other properties. Alloys with lower caratage, typically 22k, 18k, 14k or 10k, contain higher percentages of copper, or other base metals or silver or palladium in the alloy. Copper is the most commonly used base metal, yielding a redder color. Eighteen-carat gold containing 25% copper is found in antique and Russian jewelry and has a distinct, though not dominant, copper cast, creating rose gold. Fourteen-carat gold-copper alloy is nearly identical in color to certain bronze alloys, and both may be used to produce police and other badges. Blue gold can be made by alloying with iron and purple gold can be made by alloying with aluminium, although rarely done except in specialized jewelry. Blue gold is more brittle and therefore more difficult to work with when making jewelry. Fourteen and eighteen carat gold alloys with silver alone appear greenish-yellow and are referred to as green gold. White gold alloys can be made with palladium or nickel. White 18-carat gold containing 17.3% nickel, 5.5% zinc and 2.2% copper is silvery in appearance. Nickel is toxic, however, and its release from nickel white gold is controlled by legislation in Europe. Alternative white gold alloys are available based on palladium, silver and other white metals, but the palladium alloys are more expensive than those using nickel. High-carat white gold alloys are far more resistant to corrosion than are either pure silver or sterling silver. Jacques Blanc exploits the color contrasts between colored gold alloys to produce decorative skin effects.Monetary exchangeGold has been widely used throughout the world as a vehicle for monetary exchange, either by issuance and recognition of gold coins or other bare metal quantities, or through gold-convertible paper instruments by establishing gold standards in which the total value of issued money is represented in a store of gold reserves. However, the amount of gold in the world is finite and production has not grown in relation to the world's economies. Today, gold mining output is declining. With the sharp growth of economies in the 20th century, and increasing foreign exchange, the world's gold reserves and their trading market have become a small fraction of all markets and fixed exchange rates of currencies to gold were no longer sustained. Pure gold is too soft for day-to-day monetary use and is typically hardened by alloying with copper, silver or other base metals. The gold content of alloys is measured in carats (k). Pure gold is designated as 24k. Gold coins intended for circulation from 1526 into the 1930s were typically a standard 22k alloy called crown gold, for hardness. MedicineIn medieval times, gold was often seen as beneficial for the health, in the belief that something that rare and beautiful could not be anything but healthy. Even some modern esotericists and forms of alternative medicine assign metallic gold a healing power. Some gold salts do have anti-inflammatory properties and are used as pharmaceuticals in the treatment of arthritis and other similar conditions. However, only salts and radioisotopes of gold are of pharmacological value, as elemental (metallic) gold is inert to all chemicals it encounters inside the body. In modern times, injectable gold has been proven to help to reduce the pain and swelling of rheumatoid arthritis and tuberculosis. Food and drink
ToxicityPure metallic (elemental) gold is non-toxic and non-irritating when ingested and is sometimes used as a food decoration in the form of gold leaf. Metallic gold is also a component of the alcoholic drinks Goldschläger, Gold Strike, and Goldwasser. Metallic gold is approved as a food additive in the EU (E175 in the Codex Alimentarius). Although gold ion is toxic, the acceptance of metallic gold as a food additive is due to its relative chemical inertness, and resistance to being corroded or transformed into soluble salts (gold compounds) by any known chemical process which would be encountered in the human body. Gold metal was voted Allergen of the Year in 2001 by the American Contact Dermatitis Society. Gold contact allergies affect mostly women. Despite this, gold is a relatively non-potent contact allergen, in comparison with metals like nickel. Industry
The world's largest gold bar weighs 250 kg. Toi museum, Japan.
ElectronicsGold is highly conductive to electricity, and has been used for electrical wiring in some high-energy applications (only silver and copper are more conductive per volume, but gold has the advantage of corrosion resistance). For example, gold electrical wires were used during some of the Manhattan Project's atomic experiments, but large high current silver wires were used in the calutron isotope separator magnets in the project. Its good conductivity and general resistance to oxidation and corrosion in other environments (including resistance to non-chlorinated acids) has led to its widespread industrial use in the electronic era as a thin layer coating electrical connectors of all kinds, thereby ensuring good connection. For example, gold is used in the connectors of the more expensive electronics cables, such as audio, video and USB cables. The benefit of using gold over other connector metals such as tin in these applications is highly debated. Gold connectors are often criticized by audio-visual experts as unnecessary for most consumers and seen as simply a marketing ploy. However, the use of gold in other applications in electronic sliding contacts in highly humid or corrosive atmospheres, and in use for contacts with a very high failure cost (certain computers, communications equipment, spacecraft, jet aircraft engines) remains very common. Besides sliding electrical contacts, gold is also used in electrical contacts because of its resistance to corrosion, electrical conductivity, ductility and lack of toxicity. Switch contacts are generally subjected to more intense corrosion stress than are sliding contacts. Fine gold wires are used to connect semiconductor devices to their packages through a process known as wire bonding. ChemistryGold is attacked by and dissolves in alkaline solutions of potassium or sodium cyanide, and gold cyanide is the electrolyte used in commercial electroplating of gold onto base metals and electroforming. Gold chloride (chloroauric acid) solutions are used to make colloidal gold by reduction with citrate or ascorbate ions. Gold chloride and gold oxide are used to make highly valued cranberry or red-colored glass, which, like colloidal gold suspensions, contains evenly sized spherical gold nanoparticles.
GildingThe term gilding covers a number of decorative techniques for applying fine gold leaf or powder to solid surfaces such as wood, stone, or metal to give a thin coating of gold. A gilded object is described as "gilt". Where metal is gilded it was traditionally usuallysilver in the West, to make silver-gilt (or "vermeil") objects, but gilt-bronze is much used in China, and also called ormolu if it is Western. Methods of gilding include hand application and glueing, chemical gilding, and electroplating, the last also called gold plating. Parcel-gilt objects are only gilded over part of their surfaces. This may mean that all of the inside, and none of the outside, of a chalice or similar vessel is gilded, or that patterns or images are made up by using a combination of gilt and un-gilt areas. Origins and spread of gildingHerodotus mentions that the Egyptians gilded wood and metals, and many such objects have been excavated. Certain Ancient Greek statues of great prestige were chryselephantine, i.e. made of gold-plated wood (for the clothing) and ivory (for the flesh); most famously those of Zeus in Olympia and Athena Parthenos in the Parthenon. Extensive ornamental gilding was also used in the ceiling coffers of thePropylaea. Pliny the Elder informs us that the first gilding seen at Rome was after the destruction of Carthage, under the censorship of Lucius Mummius, when the Romans began to gild the ceilings of their temples and palaces, the Capitol being the first place on which this process was used. But he adds that luxury advanced on them so rapidly that in very little time you might see all, even private and poor people, gild the walls, vaults, and other parts of their dwellings. Owing to the comparative thickness of the gold leaf used in ancient gilding, the traces of it which yet remain are remarkably brilliant and solid. Fire-gilding of metal goes back at least to the 4th century BC, and was known to Pliny and Vitruvius. In Europe, silver-gilt has always been more common than gilt-bronze, but in China the opposite has been the case. The medieval Chinese also developed the gilding of porcelain, which taken up by the French and other European potters. Gilding processesModern gilding is applied to numerous and diverse surfaces and by various processes; those used in modern technology are described in gold plating. More traditional techniques still form an important part of framemaking and are sometimes still employed in general woodworking, cabinet-work, decorative painting and interior decoration, bookbinding, and ornamental leather work, and in the decoration of pottery, porcelain, and glass. Mechanical gildingMechanical gilding includes all the operations in which gold leaf is prepared, and the processes to mechanically attach the gold onto surfaces. The techniques include burnishing, water gilding and oil-gilding used by wood carvers and gilders; and the gilding operations of the house decorator, sign painter, bookbinder, the paperstainer and several others. Polished iron, steel and other metals are gilded mechanically by applying gold leaf to the metallic surface at a temperature just under red-hot, pressing the leaf on with a burnisher, then reheating when additional leaf may be laid on. The process is completed by cold burnishing. "Overlaying" or folding or hammering on gold foil or gold leaf is the simplest and most ancient method, and is mentioned in Homer's Odyssey and the Old Testament. TheRam in a Thicket of about 2600-2400 BC from Ur uses this technique on wood, with a thin layer of bitumen underneath to help adhesion. The next advances involved two simple processes. The first involves gold leaf, which is gold that is hammered or cut into very thin sheets. Gold leaf is often thinner than standard paper today, and when held to the light is semi-transparent; in ancient times it was typically about 10 times thicker than today, and perhaps half that in the Middle Ages. The object being gilded was coated with adhesive, usually gesso. "Gesso" is a substance made of finely ground gypsum or chalk mixed with glue. Once the coating of gesso had been applied, allowed to dry and smoothed. It is re-wet with glue waster or size and the gold leaf was layered on and left to dry. A second gilding process was using the gold as pigment in paint. The artist ground the gold into a fine powder and mixed it with a binder. Then the gold was applied as with any paint. Sometimes, after either gold-leafing or gold-painting, the artist would heat the piece enough to melt the gold slightly, ensuring an even coat. These techniques remained the only alternative for materials like wood, leather, and the vellum pages of illuminated manuscripts. Chemical gildingChemical gilding embraces those processes in which the gold is at some stage of chemical combination. These include: Cold gildingIn this process the gold is obtained in a state of extremely fine division, and applied by mechanical means. Cold gilding on silver is performed by a solution of gold in aqua regia, applied by dipping a linen rag into the solution, burning it, and rubbing the black and heavy ashes on the silver with the finger or a piece of leather or cork. Wet gildingWet gilding is effected by means of a dilute solution of gold chloride with twice its quantity of ether. The liquids are agitated and allowed to rest, when the ether separates and floats on the surface of the acid. The whole mixture is then poured into a funnel with a small aperture, and allowed to rest for some time, when the acid is run off and the ether separated. The ether will be found to have taken up all the gold from the acid, and may be used for gilding iron or steel, for which purpose the metal is polished with fine emery and spirits of wine. The ether is then applied with a small brush, and as it evaporates it deposits the gold, which can now be heated and polished. For small delicate figures, a pen or a fine brush may be used for laying on the ether solution. The gold chloride can also be dissolved in water in electroless plating wherein the gold is slowly reduced out of solution onto the surface to be gilded. When this technique is used on the second surface of glass and backed with silver, it is known as "Angel gilding Fire-gildingFire-gilding or Wash-gilding is a process by which an amalgam of gold is applied to metallic surfaces, the mercury being subsequentlyvolatilized, leaving a film of gold or an amalgam containing from 13 to 16% of mercury. In the preparation of the amalgam the gold must first be reduced to thin plates or grains, which are heated red hot, and thrown into previously heated mercury, until it begins to smoke. Upon stirring the mercury with an iron rod, the gold totally disappears. The proportion of mercury to gold is generally six or eight to one. When the amalgam is cold it is squeezed through chamois leather to separate the superfluous mercury; the gold, with about twice its weight of mercury, remains behind, forming a yellowish silvery mass with the consistency of butter. Gilding wax is composed of bees wax mixed with some of the following substances: red ochre, verdigris, copper scales, alum, vitriol, andborax. By this operation the color of the gilding is heightened, and the effect seems to be produced by a perfect dissipation of some mercury remaining after the former operation. The dissipation is well effected by this equable application of heat. The gilt surface is then covered over with potassium nitrate, alum or other salts, ground together, and mixed into a paste with water or weak ammonia. The piece of metal thus covered is exposed to heat, and then quenched in water. By this method its color is further improved and brought nearer to that of gold, probably by removing any particles of copper that may have been on the gilt surface. This process, when skillfully carried out, produces gilding of great solidity and beauty, but owing to the exposure of the workmen to mercurial fumes, it is very unhealthy. There is also much loss of mercury to the atmosphere, which brings extremely serious environmental concerns as well. This method of gilding metallic objects was formerly widespread, but fell into disuse as the dangers of mercury toxicity became known. Depletion gildingIn depletion gilding, a subtractive process discovered in Pre-columbian Mesoamerica, articles are fabricated by various techniques from an alloy of copper and gold, named tumbaga by the Spaniards. The surface is etched with acids, resulting in a surface of porous gold. The porous surface is then burnished down, resulting in a shiny gold surface. The results fooled the conquistadors into thinking they had massive quantities of pure gold. The results startled modern archaeologists, because at first the pieces resemble electroplated articles. Keum-boo is a special Korean technique of silver-gilding, using depletion gilding. CeramicsThe gilding of decorative ceramics has been undertaken for centuries, with the permanence and brightness of gold appealing to designers. Both porcelain and earthenware are commonly decorated with gold, and in the late 1970s it was reported that 5 tonnes of gold were used annually for the decoration of these products. Some wall tiles also have gold decoaration. Application techniques include spraying,brushing, banding machines and direct or indirect screen-printing. After application the decorated ware is fired in kiln to fuse the gold to theglaze and hence ensure its permance. The most important factors affecting coating quality are the composition of applied gold, the state of the surface before application, the thickness of the layer and the firing conditions. A number of different forms and compositions are available to apply gold to ceramic, and these include :
(mainly from Wikipedia, the free encyclopedia)
|